
Analysis of the level of ZO-1 by western blotting revealed that doxorubicin reduced the protein level in HDMECs and HCMECs. This suggests that cardiac endothelial cells are more susceptible to Herceptin than endothelial cells from other anatomical locations. Herceptin treatment caused tight junction barrier perturbment in HCMECs only. This is in agreement with the fact that doxorubicin does not effectively penetrate the BBB in patients ( Blasberg and Groothuis, 1986). The data presented in Fig. 1A-C demonstrate that doxorubicin induced tight junction barrier perturbment in HDMECs and HCMECs, but not HBMECs.

The tight junction barrier was assessed by immunofluorescence staining of the tight junction protein zona occludens-1 (ZO-1). These data suggest that cardiac microvascular injury may be an initiating and contributory event in drug-induced cardiotoxicity. Using a number of in vitro methods we showed that doxorubicin and Herceptin can affect cardiac microvascular endothelial cell barrier function leading to increased drug permeability. We were interested in the possibility that cardiotoxic drugs such as Herceptin and doxorubicin may directly affect cardiac endothelial cell function. Tubulin binding drugs, such as vincristine, have been shown to adversely affect rat cardiac microvascular endothelial cells ( Mikaelian et al., 2010), while doxorubicin has recently been shown to affect VEGF signalling in rat cardiac microvascular endothelial cells ( Chiusa et al., 2012). Whilst the majority of studies analysing the molecular mechanism of cardiotoxicity have focused on effects on cardiomyocytes, there is a growing awareness that cardiotoxic anti-cancer drugs can also adversely affect cardiac vascular function ( Chintalgattu et al., 2013 Chiusa et al., 2012). The importance of HER2 function in cardiomyocytes is highlighted by the fact that cardiotoxicity of doxorubicin is aggravated by co-administration of Herceptin, which has led to the sequential administration of these drugs in patients to reduce the severity of cardiovascular toxicity ( Gianni et al., 2007). HER2 can heterodimerise with HER3 (EGFR3) and HER4 (EGFR4) following agonist stimulation with neuregulins, which activates an intracellular signalling cascade in cardiomyocytes leading to cell survival ( Creedon et al., 2014). Gene ablation of Her2 in mice leads to embryonic lethality at embryonic day (E)9.5-10.5 due to trabeculae malformation in the heart ( Harari and Yarden, 2000). The mechanism of cardiotoxicity has been attributed to blocking normal HER2 function in cardiomyocytes ( Force et al., 2007). Despite its clinical efficacy, Herceptin has been demonstrated to lead to decreased LVEF in some patients and is classified as type II reversible cardiotoxicity ( Lemmens et al., 2007 Sandoo et al., 2014). Herceptin (trastuzumab) is a humanised monoclonal antibody that specifically targets HER2 (EGFR-2/ERBB2) ( Walshe et al., 2006), which is overexpressed in approximately 20% of breast cancers ( Baselga et al., 1998). Our data suggests that doxorubicin and Herceptin can affect tight junction formation in the cardiac microvasculature leading to increased drug permeability and adverse effects on the cardiac myocytes. HCMECs showed detectable levels of HER2 compared with the other endothelial cells suggesting that Herceptin binding to HER2 in these cells may interfere with tight junction formation. Herceptin treatment had no effect on the tight junction barrier function in human dermal and human brain microvascular endothelial cells. The effect of these drugs on the endothelial tight junction barrier was tested by analysing tight junction formation and zona occludens-1 (ZO-1) levels, revealing that Herceptin and doxorubicin are able to induce barrier perturbment and decrease barrier function in human cardiac microvascular endothelial cells (HCMECs) leading to increased permeability. Herceptin and doxorubicin are known to induce cardiotoxicity in the clinic. More recently it has become apparent that non-cardiomyocyte cells of the heart can potentially contribute to cardiotoxicity.
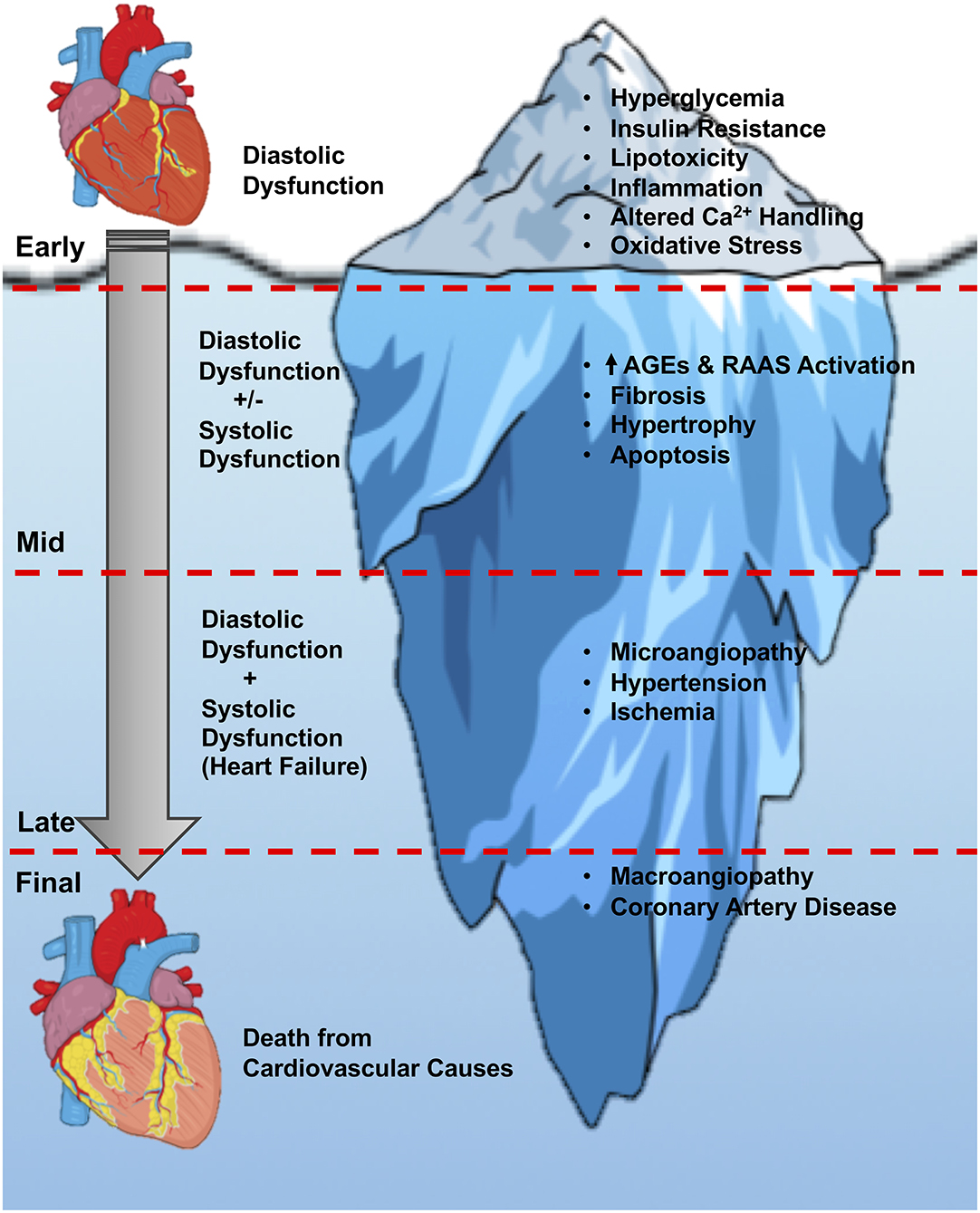
Current in vitro approaches to assess cardiotoxicity have focused on analysing cardiomyocytes. Cardiotoxicity induced by anti-cancer therapeutics is a severe, and potentially fatal, adverse reaction of the heart in response to certain drugs.


 0 kommentar(er)
0 kommentar(er)
